A Process for removing toxic heavy metals to produce the high purity NH4H2PO4 and KH2PO4 from a crude phosphoric acid - ScienceDirect
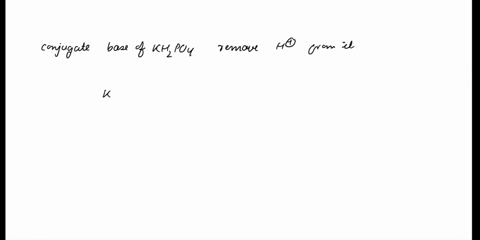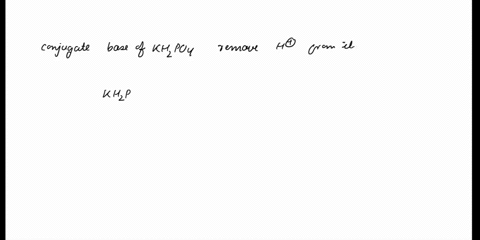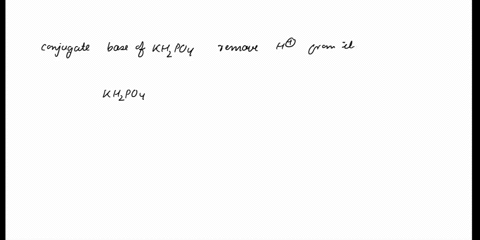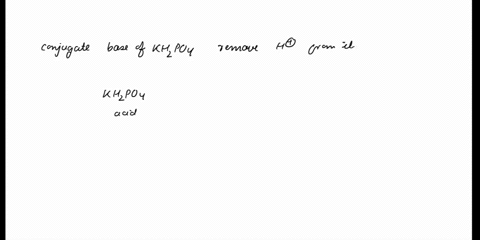
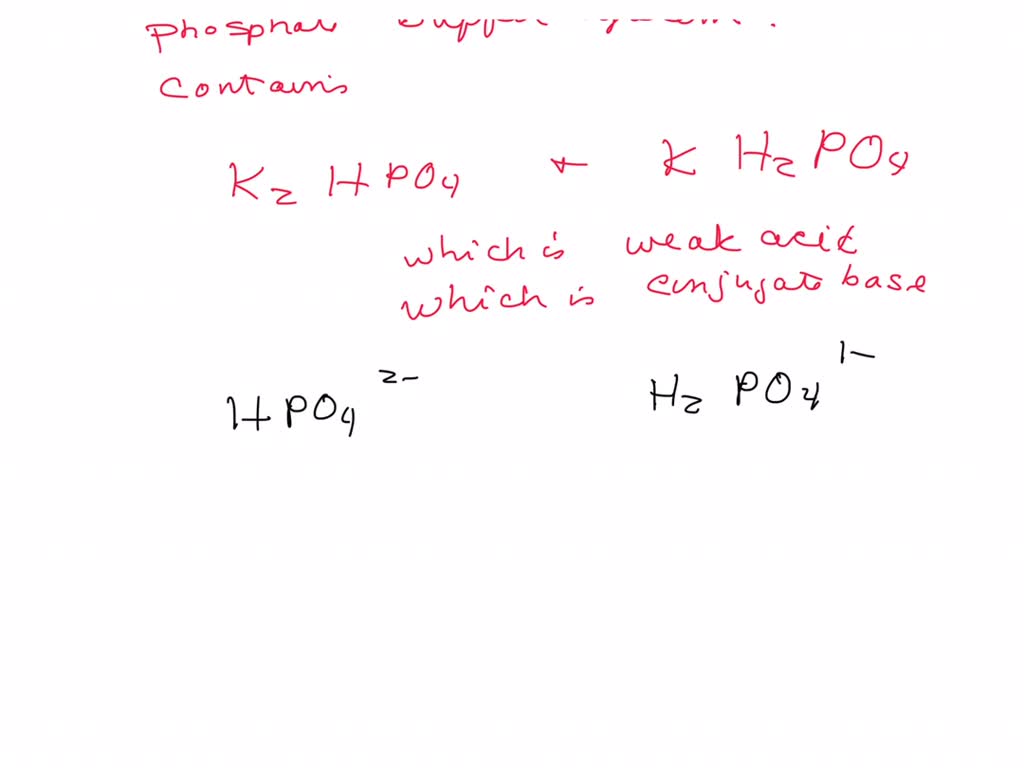
SOLVED: In the phosphate buffer system containing K2HPO4 and KH2PO4, what is the weak acid? What is its conjugate base?
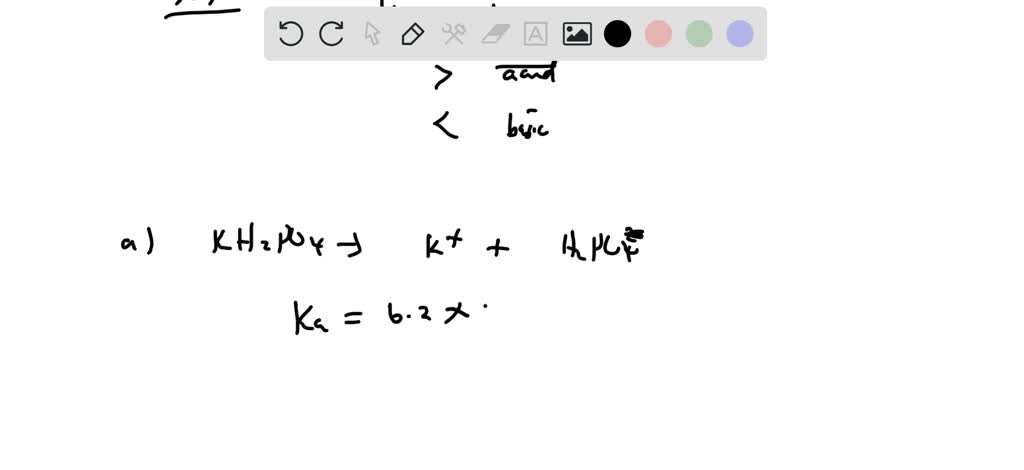
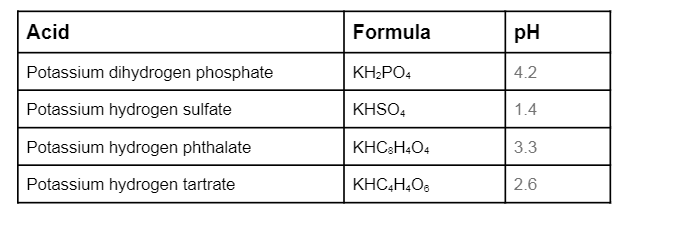
SOLVED:State whether each of the following solutions is acidic, basic, or neutral. (a) potassium dihydrogen phosphate (b) potassium hydrogen carbonate
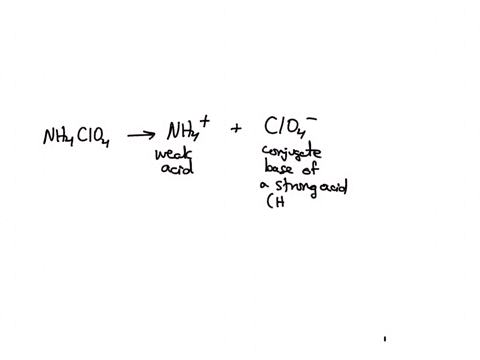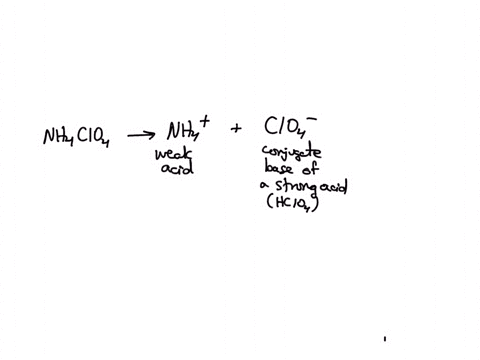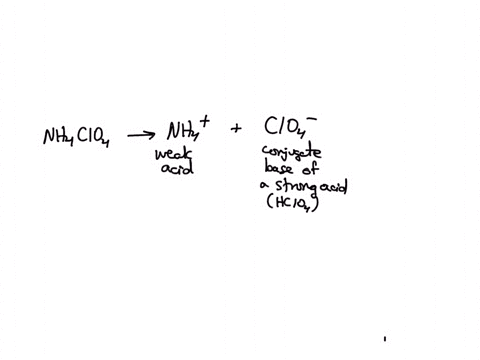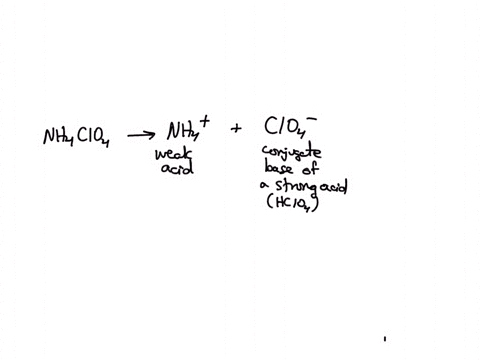
SOLVED: Polyprotic Acids (1) Is Dipotassium phosphate acidic, basic or neutral? (2) Is monopotassium phosphate (also called Potassium dihydrogen phosphate) acidic, basic or neutral? (3) Is Disodium citrate acidic, basic or neutral?

Calculate the pH of a buffer solution obtained by dissolving 25.0 g of KH2PO4(s) and 38.0 g of Na2HPO4(s) in water and then diluting to 1.00 L. | Homework.Study.com
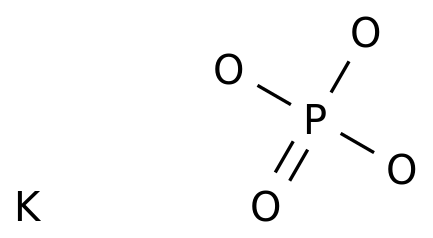

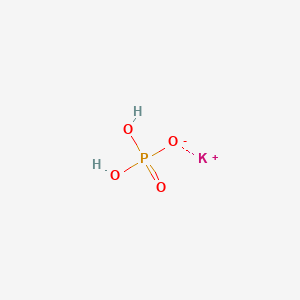


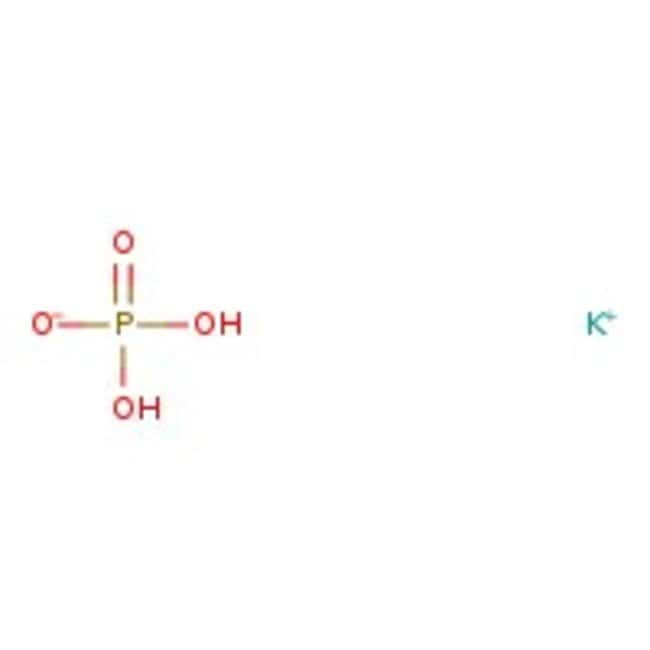

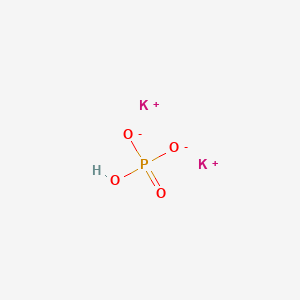
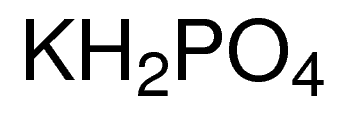

(358).jpg)